When you place a hot object in contact to a colder object, the heat from the hotter object will transfer to the colder object until they are both of the same temperature. This state is called thermal equilibrium.
This is actually how thermometers work – heat will transfer from the surroundings towards the thermometer until they are in thermal equilibrium. For alcohol and mercury thermometers, the alcohol or mercury inside the glass expands or contracts according to the temperature change, until it is at thermal equilibrium, which is when we can finally read them.
But of course, this process of exchanging heat before reaching thermal equilibrium takes time, and naturally, this time or rate is dependent on the material, and this is quantified in the thermal rate constant Tau. This rate of change is described in the following equation.

Where delta T is the difference between the initial temperature Ti and the final temperature Tf. As you can observe, the above equation is a first order linear differential equation, and with Math 121 techniques we obtain the following solution, which describes the temperature T of the material as a function of time t.
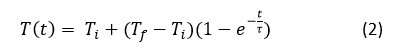
The graph of the equation above is as follows:
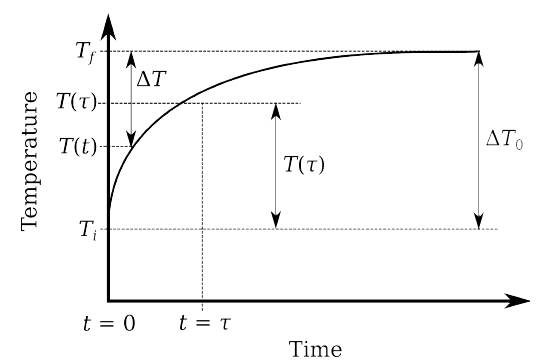
τ is actually the time it takes for the sensor to reach 63.2% of its final temperature.
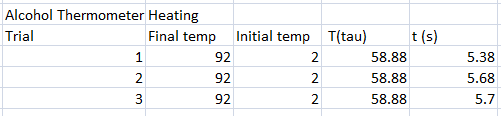
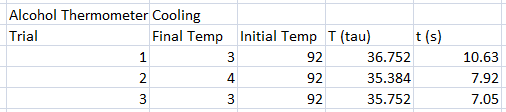
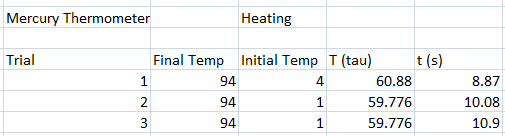
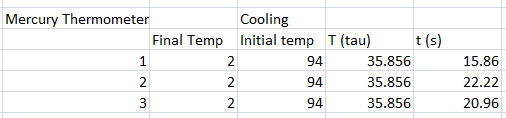
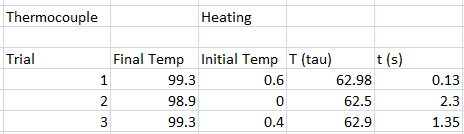
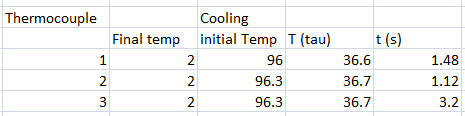
In our experiment, we tested different thermometers to determine their thermal rate constant τ. We tried three kinds of thermometers: alcohol thermometer, mercury thermometer, and a thermocouple sensor.
The following were the data for the trials, with respective τ values computed.